The question of what electrolysis is is considered in the school physics course, and for most people it is not a secret. Another thing is its importance and practical application. This process is used with great benefit in various industries and can be useful for the home craftsman.
Content
What is electrolysis?
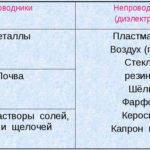
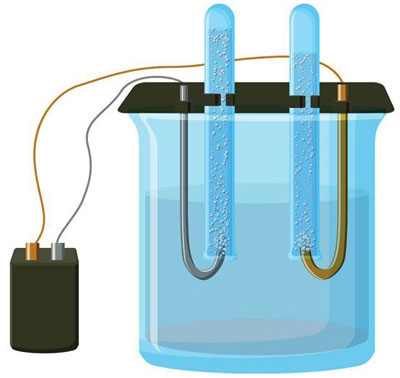
Electrolysis is a complex of specific processes in the system of electrodes and electrolyte when a direct electric current flows through it. Its mechanism is based on the occurrence of an ionic current. The electrolyte is a type 2 conductor (ionic conductivity) in which electrolytic dissociation occurs. It is associated with decomposition into ions with positive (cation) and negative (anion) charge.
The electrolysis system necessarily contains a positive (anode) and negative (cathode) electrode. When a direct electric current is applied, cations begin to move towards the cathode, and anions - towards the anode. The cations are mainly metal ions and hydrogen, and the anions are oxygen, chlorine. At the cathode, cations attach excess electrons to themselves, which ensures the occurrence of the reduction reaction Men+ + ne → Me (where n is the valency of the metal). At the anode, on the contrary, an electron is donated from the anion with an oxidative reaction taking place.
Thus, a redox process is provided in the system. It is important to consider that for its flow, appropriate energy is needed. It must be provided by an external current source.
Faraday's laws of electrolysis
The great physicist M. Faraday, with his research, made it possible not only to understand the nature of electrolysis, but also to make the necessary calculations for its implementation. In 1832, his laws appeared, linking the main parameters of the ongoing processes.
First Law
Faraday's first law states that the mass of the substance being reduced at the anode is directly proportional to the electric charge induced in the electrolyte: m = kq = k*I*t, where q is the charge, k is the coefficient or electrochemical equivalent of the substance, I is the strength of the current flowing through electrolyte, t is the current passage time.

Second law
Faraday's second law made it possible to determine the coefficient of proportionality k. It sounds like this: the electrochemical equivalent of any substance is directly proportional to its molar mass and inversely proportional to valency. The law is expressed as:

k = 1/F*A/z, where F is the Faraday constant, A is the molar mass of the substance, z is its chemical valence.
Taking into account both laws, it is possible to derive the final formula for calculating the mass deposited on the electrode of the substance: m = A*I*t/(n*F), where n is the number of electrons involved in electrolysis. Usually n corresponds to the charge of the ion. From a practical point of view, the connection between the mass of a substance and the applied current is important, which makes it possible to control the process by changing its strength.
Melt electrolysis
One of the options for electrolysis is the use of a melt as an electrolyte. In this case, only melt ions participate in the electrolysis process. A classic example is the electrolysis of molten salt NaCl (salt). Negative ions rush to the anode, which means that gas is released (Cl). Metal reduction will occur at the cathode, i.e. deposition of pure Na formed from positive ions that have attracted excess electrons. Other metals can be obtained similarly (K, Ca, Li, etc.) from the massacre of the corresponding salts.
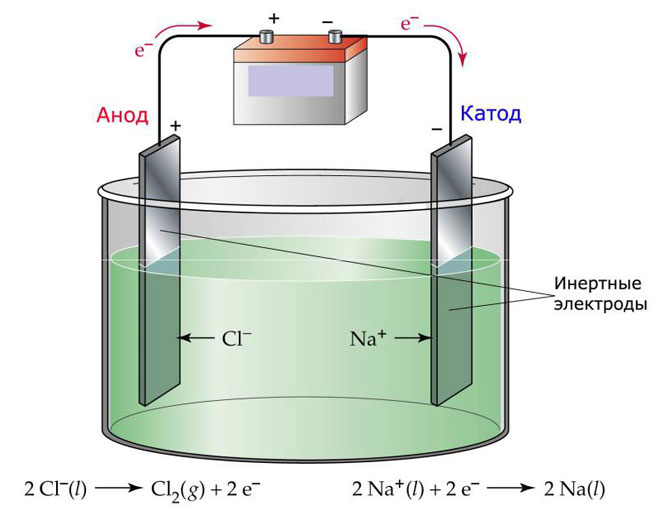
During electrolysis in a melt, the electrodes do not undergo dissolution, but participate only as a current source. In their manufacture, you can use metal, graphite, some semiconductors. It is important that the material has sufficient conductivity. One of the most common materials is copper.
Features of electrolysis in solutions
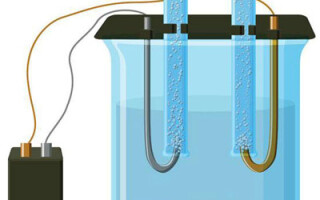
Electrolysis in an aqueous solution differs significantly from a melt. Three competing processes take place here: water oxidation with oxygen evolution, anion oxidation, and anodic dissolution of the metal. The ions of water, electrolyte and anode are involved in the process.Accordingly, reduction of hydrogen, electrolyte cations, and anode metal can occur at the cathode.

The possibility of these competing processes occurring depends on the magnitude of the electrical potentials of the system. Only the process that requires less external energy will proceed. Consequently, cations with the maximum electrode potential will be reduced at the cathode, and anions with the lowest potential will be oxidized at the anode. The electrode potential of hydrogen is taken as "0". For example, for potassium it is (-2.93 V), sodium - (-2.71 V), lead (-0.13V), while silver has (+0.8 V).
Electrolysis in gases
Gas can play the role of an electrolyte only in the presence of an ionizer. In this case, the current passing through the ionized medium causes the necessary process on the electrodes. However, Faraday's laws do not apply to gas electrolysis. For its implementation, the following conditions are necessary:
- Without artificial ionization of the gas, neither high voltage nor high current will help.
- Only acids that do not contain oxygen and are in a gaseous state, and some gases are suitable for electrolysis.
Important! When the necessary conditions are met, the process proceeds similarly to electrolysis in a liquid electrolyte.
Features of the processes occurring at the cathode and anode
For the practical application of electrolysis, it is important to understand what happens at both electrodes when an electric current is applied. Typical processes are:
- Cathode. Positively charged ions rush to it. Here, the reduction of metals or the evolution of hydrogen takes place. There are several categories of metals according to cationic activity.Metals such as Li, K, Ba, St, Ca, Na, Mg, Be, Al are well reduced only from molten salts. If a solution is used, then hydrogen is released due to the electrolysis of water. It is possible to achieve reduction in solution, but with a sufficient concentration of cations, for the following metals - Mn, Cr, Zn, Fe, Cd, Ni, Ti, Co, Mo, Sn, Pb. The process proceeds most easily for Ag, Cu, Bi, Pt, Au, Hg.
- Anode. Negatively charged ions enter this electrode. Oxidized, they take electrons from the metal, which leads to their anodic dissolution, i.e. transition into positively charged ions, which are sent to the cathode. Anions are also classified according to their activity. Such anions PO4, CO3, SO4, NO3, NO2, ClO4, F can be discharged only from melts. In aqueous solutions, it is not they that undergo electrolysis, but water with the release of oxygen. Anions such as OH, Cl, I, S, Br react most easily.

When ensuring electrolysis, it is important to take into account the tendency of the electrode material to oxidize. In this regard, inert and active anodes stand out. Inert electrodes are made of graphite, carbon or platinum and do not participate in the supply of ions.
Factors affecting the electrolysis process
The electrolysis process depends on the following factors:
- Electrolyte composition. Various impurities have a significant effect. They are divided into 3 types - cations, anions and organics. Substances can be more or less negative than the base metal, which interferes with the process. Among organic impurities, pollutants (eg oils) and surfactants stand out. Their concentration has maximum permissible values.
- current density. In accordance with Faraday's laws, the mass of the deposited substance increases with increasing current strength. However, unfavorable circumstances arise - concentrated polarization, increased voltage, intense heating of the electrolyte. With this in mind, there are optimal current density values for each specific case.
- electrolyte pH. The acidity of the environment is also selected taking into account metals. For example, the optimal value of electrolyte acidity for zinc is 140 g/cu.dm.
- Electrolyte temperature. It has an ambiguous effect. With an increase in temperature, the rate of electrolysis increases, but the activity of impurities also increases. There is an optimum temperature for every process. Usually it is in the range of 38-45 degrees.
Important! Electrolysis can be accelerated or slowed down by various influences and the choice of electrolyte composition. Each application has its own regimen, which must be strictly observed.
Where is electrolysis used?
Electrolysis is used in many areas. There are several main areas of use for obtaining practical results.
Electroplating
A thin, durable plating of metal can be applied by electrolysis. The product to be coated is installed in the bath in the form of a cathode, and the electrolyte contains a salt of the desired metal. So you can cover the steel with zinc, chromium or tin.

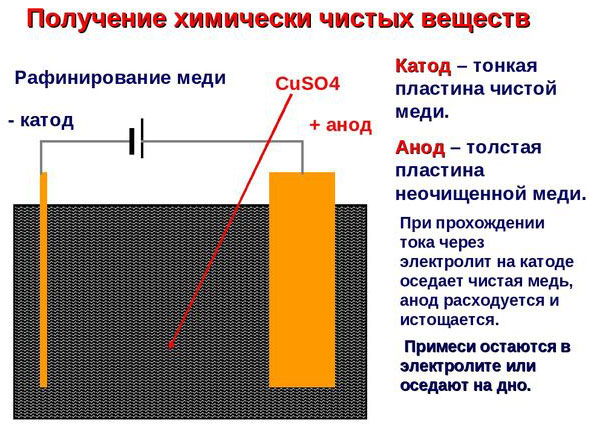
Electrorefining - copper refining
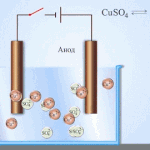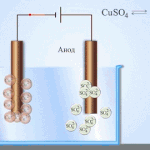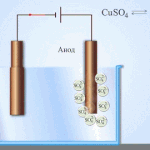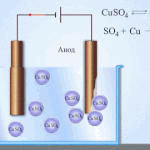
An example of electrical cleaning can be the following option: cathode - pure copper anode - copper with impurities, electrolyte - an aqueous solution of copper sulfate. Copper from the anode passes into ions and settles in the cathode already without impurities.
Metal mining
To obtain metals from salts, they are transferred to the melt, and then electrolysis is provided in it. Such a method is quite effective for obtaining aluminum from bauxites, sodium and potassium.

Anodizing
In this process, the coating is made from non-metallic compounds. A classic example is aluminum anodizing. The aluminum part is installed as an anode. The electrolyte is a solution of sulfuric acid. As a result of electrolysis, a layer of aluminum oxide is deposited on the anode, which has protective and decorative properties. These technologies are widely used in various industries. You can carry out the processes with your own hands in compliance with safety regulations.
Energy costs
Electrolysis requires high energy costs. The process will be of practical value if the anode current is sufficient, and for this it is necessary to apply a significant direct current from the power source. In addition, when it is carried out, side voltage losses occur - anode and cathode overvoltage, losses in the electrolyte due to its resistance. The efficiency of the installation is determined by relating the power of energy consumption to a unit of useful mass of the obtained substance.
Electrolysis has long been used in industry with high efficiency. Anodized and electroplated coatings have become commonplace in everyday life, and mining and beneficiation of materials helps extract many metals from ore. The process can be planned and calculated, knowing its main patterns.
Similar articles: