An electric current source is a device with which an electric current is created in a closed electrical circuit. At present, a large number of types of such sources have been invented. Each type is used for specific purposes.
Content
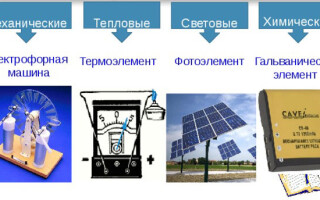
Types of electric current sources
There are the following types of electric current sources:
- mechanical;
- thermal;
- light;
- chemical.
Mechanical sources
These sources convert mechanical energy into electrical energy. The transformation is carried out in special devices - generators. The main generators are turbo generators, where the electric machine is driven by a gas or steam flow, and hydro generators, which convert the energy of falling water into electricity. Most of the electricity on Earth is produced precisely by mechanical converters.

Heat sources
Here, thermal energy is converted into electricity. The occurrence of an electric current is due to the temperature difference between two pairs of contacting metals or semiconductors - thermocouples. In this case, charged particles are transferred from a heated area to a cold one. The magnitude of the current depends directly on the temperature difference: the greater this difference, the greater the electric current. Semiconductor-based thermocouples give a thermoelectric power 1000 times greater than bimetallic ones, so current sources can be made from them. Metal thermocouples are used only to measure temperature.
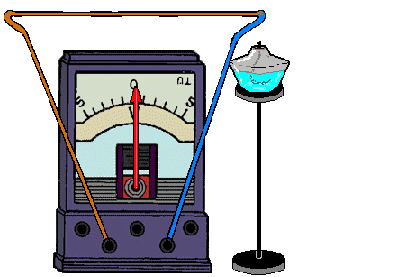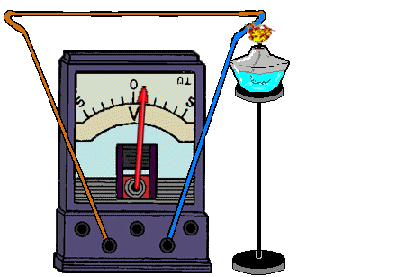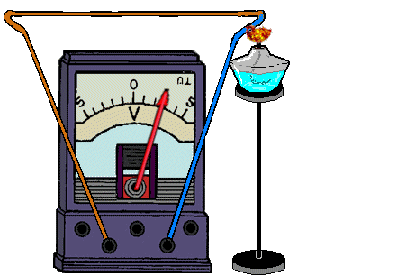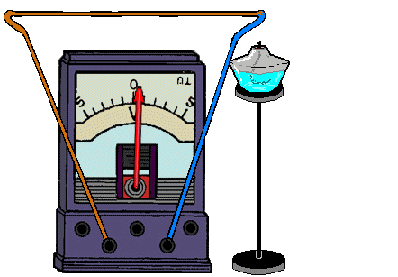
REFERENCE! To get a thermocouple, you need to connect 2 different metals.
At present, new elements have been developed based on the conversion of heat released during the natural decay of radioactive isotopes. Such elements are called radioisotope thermoelectric generator. In spacecraft, a generator using the plutonium-238 isotope has proven itself well. It gives a power of 470 W at a voltage of 30 V. Since the half-life of this isotope is 87.7 years, the life of the generator is very long. A bimetal thermocouple is used to convert heat into electricity.
light sources
With the development of semiconductor physics at the end of the 20th century, new current sources appeared - solar batteries, in which light energy is converted into electrical energy. They use the property of semiconductors to produce voltage when exposed to a light flux. This effect is especially strong in silicon semiconductors. But still, the efficiency of such elements does not exceed 15%.Solar panels have become indispensable in the space industry, and have begun to be used in everyday life. The price of such power supplies is constantly decreasing, but remains quite high: about 100 rubles per 1 watt of power.

Chemical Sources
All chemical sources can be divided into 3 groups:
- Galvanic
- Batteries
- Thermal
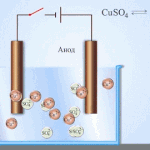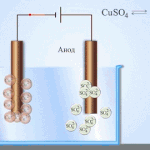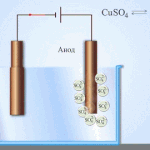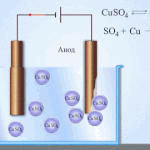
Galvanic cells work on the basis of the interaction of two different metals placed in an electrolyte. Various chemical elements and their compounds can serve as pairs of metals and an electrolyte. The type and characteristics of the element depend on this.
IMPORTANT! Galvanic cells are used only once, i.e. Once discharged, they cannot be recovered.
There are 3 types of galvanic sources (or batteries):
- Salt;
- Alkaline;
- Lithium.
Salt, or otherwise "dry", batteries use a paste-like electrolyte from a salt of a metal, placed in a zinc cup. The cathode is a graphite-manganese rod located in the center of the cup. Cheap materials and ease of manufacture of such batteries made them the cheapest of all. But in terms of characteristics, they are significantly inferior to alkaline and lithium ones.

Alkaline batteries use a paste solution of alkali, potassium hydroxide, as the electrolyte. The zinc anode was replaced with powdered zinc, which made it possible to increase the current output by the element and the operating time. These elements serve 1.5 times longer than salt ones.
In a lithium cell, the anode is made of lithium, an alkali metal, which greatly increased the duration of operation. But at the same time, the price increased due to the relative high cost of lithium. In addition, a lithium battery may have a different voltage depending on the cathode material.They produce batteries with a voltage of 1.5 V to 3.7 V.
Batteries are sources of electric current that can be subjected to many charge-discharge cycles. The main types of batteries are:
- Lead acid;
- Lithium-ion;
- Nickel-cadmium.
Lead-acid batteries consist of lead plates immersed in a solution of sulfuric acid. When an external electrical circuit is closed, a chemical reaction occurs, as a result of which lead is converted to lead sulfate at the cathode and anode, and water is also formed. During charging, lead sulfate at the anode is reduced to lead, and at the cathode to lead dioxide.

REFERENCE! One element of a lead-zinc battery generates a voltage of 2 V. By connecting the elements in series, you can get any voltage that is a multiple of 2. For example, in car batteries, the voltage is 12 V, because. connected 6 elements.
The lithium-ion battery got its name from the fact that lithium ions serve as a carrier of electricity in the electrolyte. The ions originate at the cathode, which is made of lithium salt on an aluminum foil substrate. The anode is made of various materials: graphite, cobalt oxides and other compounds on a copper foil substrate.
The voltage, depending on the components used, can be from 3 V to 4.2 V. Due to the low self-discharge and a large number of charge-discharge cycles, lithium-ion batteries have become very popular in household appliances.
IMPORTANT! Lithium-ion batteries are very sensitive to overcharging.Therefore, to charge them, you need to use chargers designed only for them, which have built-in special circuits that prevent overcharging. Otherwise, the battery may be destroyed and ignite.

In nickel-cadmium batteries, the cathode is made of nickel salt on a steel mesh, the anode is made of cadmium salt on a steel mesh, and the electrolyte is a mixture of lithium hydroxide and potassium hydroxide. The nominal voltage of such a battery is 1.37 V. It can withstand from 100 to 900 charge-discharge cycles.
REFERENCE! Nickel-cadmium batteries can be stored in a discharged state, unlike lithium-ion ones.
Thermal chemical elements serve as backup power sources. They give excellent characteristics in terms of specific current density, but have a short service life (up to 1 hour). They are mainly used in rocket technology, where reliability and short-term operation are needed.
IMPORTANT! Initially, thermal chemical sources cannot produce an electric current. In them, the electrolyte is contained in a solid state, and to bring the battery into working condition, heating to 500-600 ° C is necessary. Such heating is carried out by a special pyrotechnic mixture, which ignites at the right time.
The difference between a real source and an ideal one
An ideal source, according to the laws of physics, must have infinite internal resistance in order to ensure a constant electric current in the load. Real sources have a finite internal resistance, which means that the current depends on both the external load and the internal resistance.
Here is a brief summary of the variety of modern sources of electric current. As can be seen from the review, to date, an impressive number of sources have been created with characteristics suitable for any application.
Similar articles: