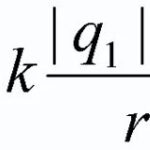Charges interact with each other in different media with different forces, determined by Coulomb's law. The properties of these media are determined by a quantity called the permittivity.

Content
What is dielectric constant
According to Coulomb's law, two fixed point charges q1 and q2 in vacuum interact with each other with the force given by the formula Fclass=((1/4)*π*ε)*(|q1|*|q2|/r2), where:
- Fclass is the Coulomb force, N;
- q1, q2 are charge modules, C;
- r is the distance between charges, m;
- ε0 - electrical constant, 8.85 * 10-12 F/m (Farad per meter).
If the interaction does not take place in a vacuum, the formula includes another quantity that determines the influence of matter on the Coulomb force, and the Coulomb law is written as follows:
F=((1/4)*π* ε* ε)*(|q1|*|q2|/r2).
This value is denoted by the Greek letter ε (epsilon), it is dimensionless (has no unit of measure). Dielectric permittivity is the coefficient of attenuation of the interaction of charges in a substance.
Often in physics, the permittivity is used in conjunction with the electrical constant, in which case it is convenient to introduce the concept of absolute permittivity. It is denoted by εa and is equal to εa= ε*e. In this case, the absolute permeability has the dimension F/m. Ordinary permeability ε is also called relative to distinguish it from εa.
The nature of the permittivity
The nature of the permittivity is based on the phenomenon of polarization under the action of an electric field. Most substances are generally electrically neutral, although they contain charged particles. These particles are located randomly in the mass of matter and their electric fields, on average, neutralize each other.
In dielectrics, there are mainly bound charges (they are called dipoles). These dipoles conventionally represent bundles of two dissimilar particles, which are spontaneously oriented along the thickness of the dielectric and, on average, create a zero electric field strength. Under the action of an external field, the dipoles tend to orient themselves according to the applied force. As a result, an additional electric field is created. Similar phenomena also occur in nonpolar dielectrics.
In conductors, the processes are similar, only there are free charges, which are separated under the action of an external field and also create their own electric field. This field is directed towards the external one, screens the charges and reduces the strength of their interaction.The greater the ability of a substance to polarize, the higher ε.
Dielectric constant of various substances
Different substances have different dielectric constants. The value of ε for some of them is given in Table 1. It is obvious that these values are greater than unity, so the interaction of charges, in comparison with vacuum, always decreases. It should also be noted that for air ε is slightly more than unity, so the interaction of charges in air practically does not differ from the interaction in vacuum.
Table 1. Values of electrical permeability for various substances.
| Substance | The dielectric constant |
|---|---|
| Bakelite | 4,5 |
| Paper | 2,0..3,5 |
| Water | 81 (at +20 degrees C) |
| Air | 1,0002 |
| Germanium | 16 |
| Getinax | 5..6 |
| Wood | 2.7..7.5 (various grades) |
| Radio engineering ceramics | 10..200 |
| Mica | 5,7..11,5 |
| Glass | 7 |
| Textolite | 7,5 |
| Polystyrene | 2,5 |
| PVC | 3 |
| Fluoroplast | 2,1 |
| Amber | 2,7 |
Dielectric constant and capacitance of the capacitor
Knowing the value of ε is important in practice, for example, when creating electrical capacitors. Them capacity depends on the geometric dimensions of the plates, the distance between them, and the permittivity of the dielectric.
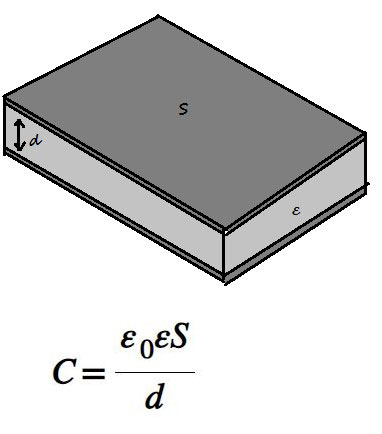
If you need to get capacitor increased capacity, then an increase in the area of the plates leads to an increase in dimensions. There are also practical limits to reducing the distance between the electrodes. In this case, the use of an insulator with increased dielectric constant can help. If you use a material with a higher ε, you can multiply reduce the size of the plates or increase the distance between them without loss electrical capacity.
Substances called ferroelectrics are distinguished into a separate category, in which, under certain conditions, spontaneous polarization occurs.In the area under consideration, they are characterized by two points:
- large values of dielectric permittivity (typical values - from hundreds to several thousand);
- the ability to control the value of the dielectric constant by changing the external electric field.
These properties are used for the manufacture of high-capacity capacitors (due to the increased value of the dielectric constant of the insulator) with small weight and size indicators.
Such devices work only in low-frequency AC circuits - as the frequency increases, their dielectric constant decreases. Another application of ferroelectrics is variable capacitors, whose characteristics change under the influence of an applied electric field with varying parameters.
Dielectric Constant and Dielectric Loss
Also, the losses in the dielectric depend on the value of the dielectric constant - this is the part of the energy that is lost in the dielectric to heat it. To describe these losses, the parameter tan δ is usually used - the tangent of the dielectric loss angle. It characterizes the power of dielectric losses in a capacitor, in which the dielectric is made of a material with an available tg δ. And the specific power loss for each substance is determined by the formula p=E2*ώ*ε*ε*tg δ, where:
- p is the specific power loss, W;
- ώ=2*π*f is the circular frequency of the electric field;
- E is the electric field strength, V/m.
Obviously, the higher the dielectric constant, the higher the losses in the dielectric, all other things being equal.
Dependence of the permittivity on external factors
It should be noted that the value of the permittivity depends on the frequency of the electric field (in this case, on the frequency of the voltage applied to the plates). As the frequency increases, the value of ε decreases for many substances. This effect is pronounced for polar dielectrics. This phenomenon can be explained by the fact that the charges (dipoles) cease to have time to follow the field. For substances that are characterized by ionic or electronic polarization, the dependence of the permittivity on frequency is small.
Therefore, the selection of materials for making a capacitor dielectric is so important. What works at low frequencies will not necessarily provide good isolation at high frequencies. Most often, non-polar dielectrics are used as an insulator at HF.
Also, the dielectric constant depends on temperature, and in different substances in different ways. For nonpolar dielectrics, it decreases with increasing temperature. In this case, for capacitors made using such an insulator, they speak of a negative temperature coefficient of capacitance (TKE) - capacity decreases with increasing temperature following ε. For other substances, the permeability increases with increasing temperature, and capacitors with a positive TKE can be obtained. By including capacitors with opposite TKE in a pair, you can get a thermally stable capacitance.
Understanding the essence and knowledge of the value of the permittivity of various substances is important for practical purposes. And the ability to control the level of dielectric constant provides additional technical perspectives.
Similar articles: