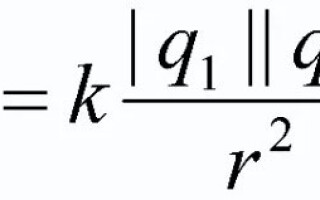Between charged bodies there is an interaction force due to which they can attract or repel each other. Coulomb's law describes this force, shows the degree of its action, depending on the size and shape of the body itself. This physical law will be discussed in this article.
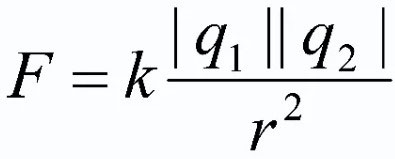
Content
- 1 Stationary point charges
- 2 Torsion balance of Charles Coulomb
- 3 Proportionality factor k and electrical constant
- 4 The direction of the Coulomb force and the vector form of the formula
- 5 Where Coulomb's law is applied in practice
- 6 Direction of forces in Coulomb's law
- 7 History of the discovery of the law
Stationary point charges
Coulomb's law applies to stationary bodies that are much smaller than their distance from other objects. A point electric charge is concentrated on such bodies. When solving physical problems, the dimensions of the considered bodies are neglected, because they don't really matter.
In practice, point charges at rest are depicted as follows:


In this case q1 and q2 - this is positive electric charges, and the Coulomb force acts on them (not shown in the figure). The size of point features does not matter.
Note! Charges at rest are located at a given distance from each other, which in problems is usually denoted by the letter r. Further in the article, these charges will be considered in a vacuum.
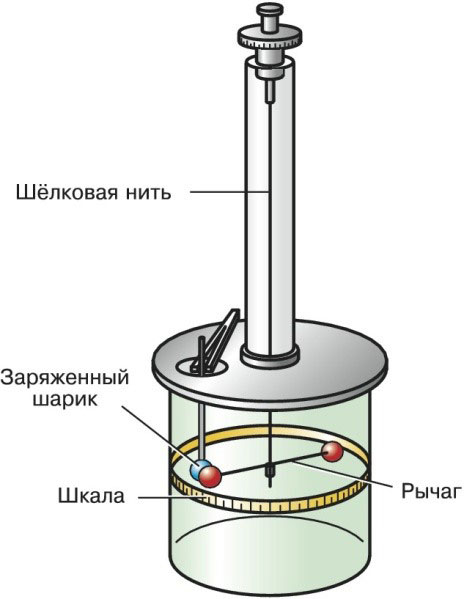
Torsion balance of Charles Coulomb
This device, developed by Coulomb in 1777, helped to deduce the dependence of the force later named after him. With its help, the interaction of point charges, as well as magnetic poles, is studied.
A torsion balance has a small silk thread located in a vertical plane from which a balanced lever hangs. Point charges are located at the ends of the lever.
Under the action of external forces, the lever begins to move horizontally. The lever will move in the plane until it is balanced by the elastic force of the thread.
In the process of movement, the lever deviates from the vertical axis by a certain angle. It is taken as d and is called the angle of rotation. Knowing the value of this parameter, it is possible to find the torque of the arising forces.
The torsion balance of Charles Coulomb looks like this:
Proportionality factor k and electrical constant 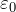
In the formula of Coulomb's law there are parameters k - the coefficient of proportionality or ![]() is the electrical constant. Electrical constant
is the electrical constant. Electrical constant ![]() presented in many reference books, textbooks, the Internet, and it does not need to be counted! Vacuum proportionality factor based on
presented in many reference books, textbooks, the Internet, and it does not need to be counted! Vacuum proportionality factor based on ![]() can be found by the well-known formula:
can be found by the well-known formula:
![]()
Here ![]() is the electrical constant,
is the electrical constant,
![]() - Pi,
- Pi,
![]() is the coefficient of proportionality in vacuum.
is the coefficient of proportionality in vacuum.
Additional Information! Without knowing the parameters presented above, it will not work to find the force of interaction between two point electric charges.
Formulation and formula of Coulomb's law
To summarize the above, it is necessary to give the official formulation of the main law of electrostatics. It takes the form:
The force of interaction of two point charges at rest in vacuum is directly proportional to the product of these charges and inversely proportional to the square of the distance between them. Moreover, the product of charges must be taken modulo!
![]()
In this formula q1 and q2 are point charges, considered bodies; r2 - the distance on the plane between these bodies, taken in the square; k is the coefficient of proportionality (![]() for vacuum).
for vacuum).
The direction of the Coulomb force and the vector form of the formula
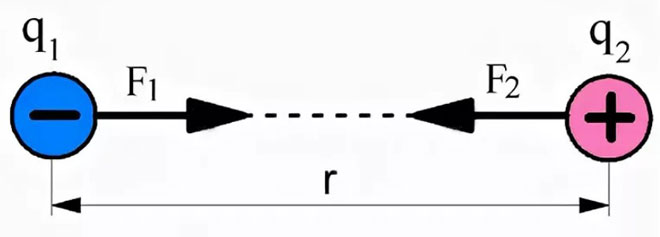
For a complete understanding of the formula, Coulomb's law can be visualized:
F1,2 - the force of interaction of the first charge with respect to the second.
F2,1 - the force of interaction of the second charge in relation to the first.
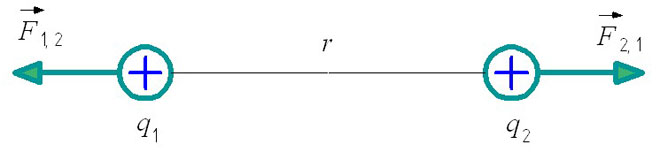
Also, when solving problems of electrostatics, it is necessary to take into account an important rule: electric charges of the same name repel, and opposite charges attract. The location of the interaction forces in the figure depends on this.
If opposite charges are considered, then the forces of their interaction will be directed towards each other, depicting their attraction.
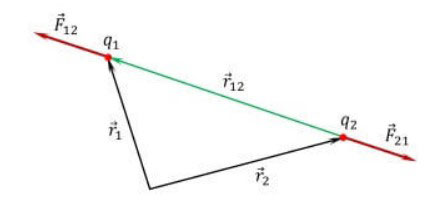
The formula of the basic law of electrostatics in vector form can be represented as follows:
![]()
![]() is the force acting on the point charge q1, from the side of the charge q2,
is the force acting on the point charge q1, from the side of the charge q2,
![]() is the radius vector connecting the charge q2 with the charge q1,
is the radius vector connecting the charge q2 with the charge q1,
![]()
Important! Having written the formula in vector form, the interacting forces of two point electric charges will need to be projected onto the axis in order to correctly put the signs. This action is a formality and is often performed mentally without any notes.
Where Coulomb's law is applied in practice
The basic law of electrostatics is the most important discovery of Charles Coulomb, which has found its application in many areas.
The works of the famous physicist were used in the process of inventing various devices, devices, apparatuses. For example, a lightning rod.
With the help of a lightning rod, residential buildings and buildings are protected from lightning during a thunderstorm. Thus, the degree of protection of electrical equipment is increased.
The lightning rod works according to the following principle: during a thunderstorm, strong induction charges gradually begin to accumulate on the ground, which rise up and are attracted to the clouds. In this case, a rather large electric field is formed on the ground. Near the lightning rod, the electric field becomes stronger, due to which a corona electric charge is ignited from the tip of the device.
Further, the charge formed on the ground begins to be attracted to the charge of the cloud with the opposite sign, as it should be according to Charles Coulomb's law. After that, the air goes through the process of ionization, and the electric field strength becomes less near the end of the lightning rod. Thus, the risk of lightning entering the building is minimal.
Note! If a blow hits the building on which the lightning rod is installed, then there will be no fire, and all the energy will go into the ground.
Based on Coulomb's law, a device called the "Particle Accelerator" was developed, which is in great demand today.
In this device, a strong electric field is created, which increases the energy of particles falling into it.
Direction of forces in Coulomb's law
As mentioned above, the direction of the interacting forces of two point electric charges depends on their polarity. Those. Charges of the same name will repel, and charges of opposite charges will attract.
Coulomb forces can also be called the radius vector, since they are directed along the line drawn between them.
In some physical problems, bodies of complex shape are given, which cannot be taken for a point electric charge, i.e. ignore its size. In this situation, the body under consideration must be divided into several small parts and each part must be calculated separately, using Coulomb's law.
The force vectors obtained during splitting are summarized according to the rules of algebra and geometry. As a result, the resultant force is obtained, which will be the answer for this problem. This method of solving is often called the triangle method.
History of the discovery of the law
The interactions of two point charges by the law considered above were first proved in 1785 by Charles Coulomb. The physicist managed to prove the veracity of the formulated law using torsion balances, the principle of operation of which was also presented in the article.
Coulomb also proved that there is no electric charge inside a spherical capacitor. So he came to the conclusion that the magnitude of electrostatic forces can be changed by changing the distance between the bodies under consideration.
Thus, Coulomb's law is still the most important law of electrostatics, on the basis of which many of the greatest discoveries have been made. Within the framework of this article, the official wording of the law was presented, as well as its constituent parts were described in detail.
Similar articles: